The Crossword Solver found 30 answers to “extract obtained by distillation (7)”, 7 letters crossword clue. The Crossword Solver finds answers to classic crosswords and cryptic crossword puzzles. Enter the length or pattern for better results. Click the answer to find similar crossword clues . A clue is required.. The best results for high-quality distillates were obtained at 85 °C (EVT) and confirmed that linalyl acetate content increased from 51.54 mg/g (initial Bulgarian lavender extract, L-Bg-E) and 89.53 mg/g (initial Polish lavender extract, L-Pl-E) to 118.41 and 185.42 mg/g, respectively, corresponding to increases of 2.3 and 2.1 times in both.

11+ Examples of Evaporation in our daily life (Explained!) Teachoo
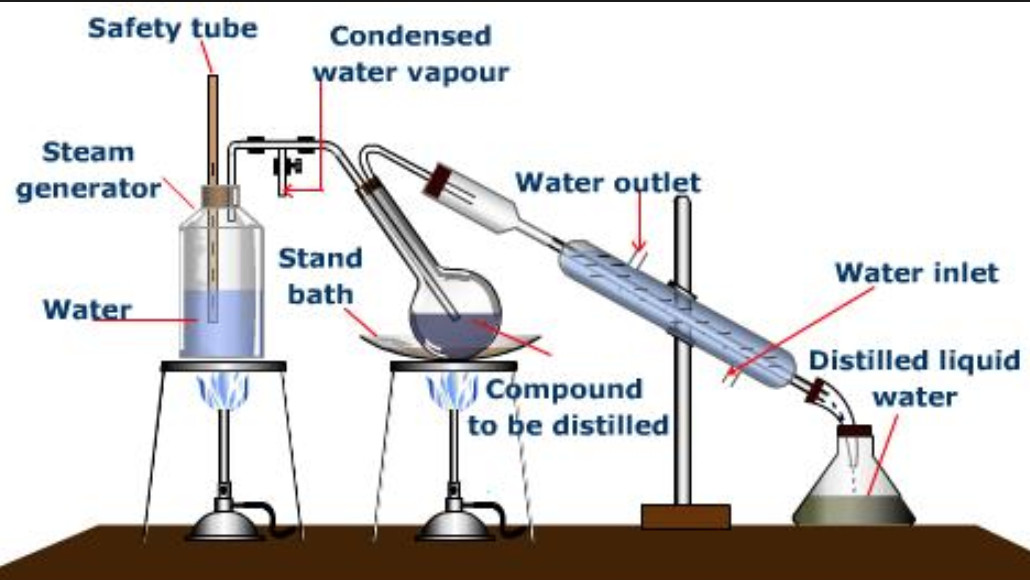
Purification of Organic Compounds Chemistry, Class 11, Organic Chemistry Some Basic
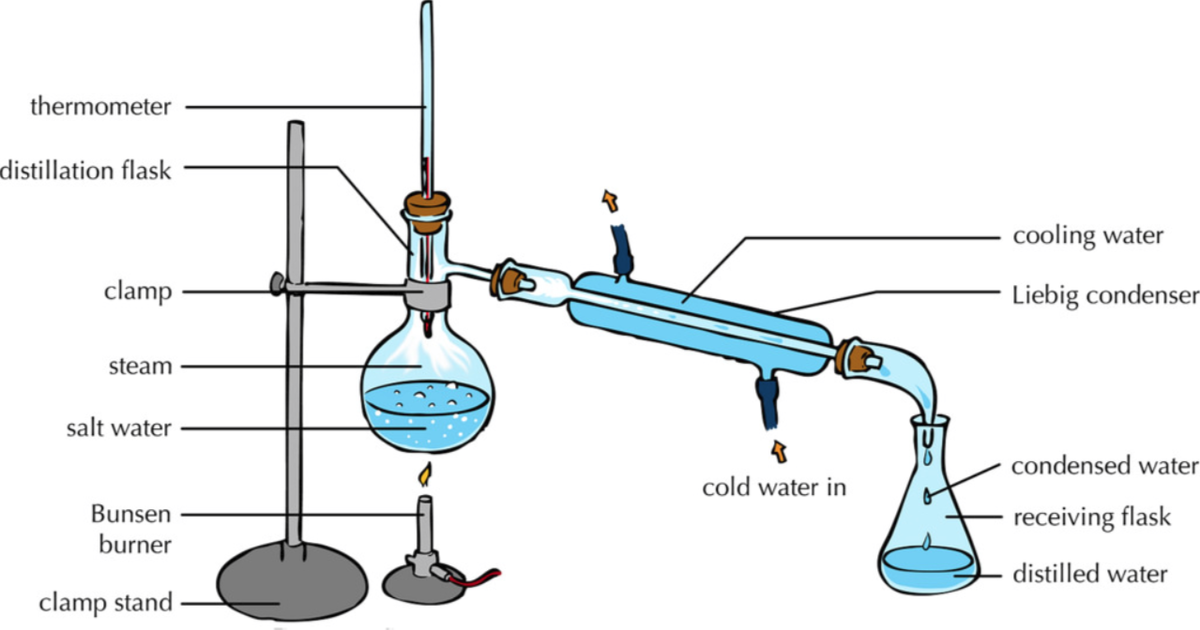
Distillation Key Stage Wiki

Distillation of oils from plants. YouTube
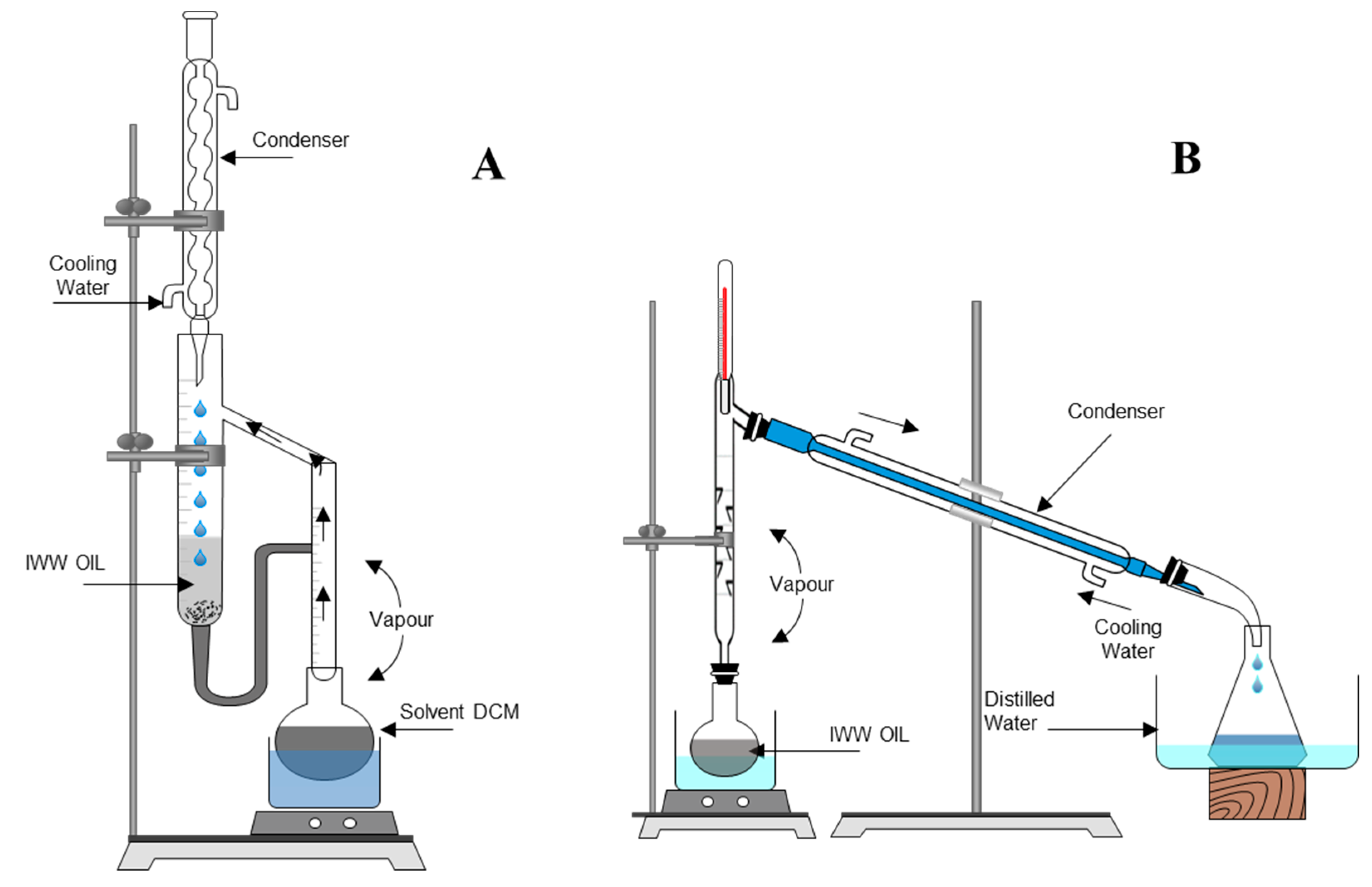
Water Free FullText LiquidLiquid Continuous Extraction and Fractional Distillation for the

Distillation Equipment Wiped Film and Short Path

Steam Distillation , Method To Extract and Isolate Essential Oils from Lavender Flowers Stock

Water distillation process as physics method for pure water extraction outline diagram. Labeled
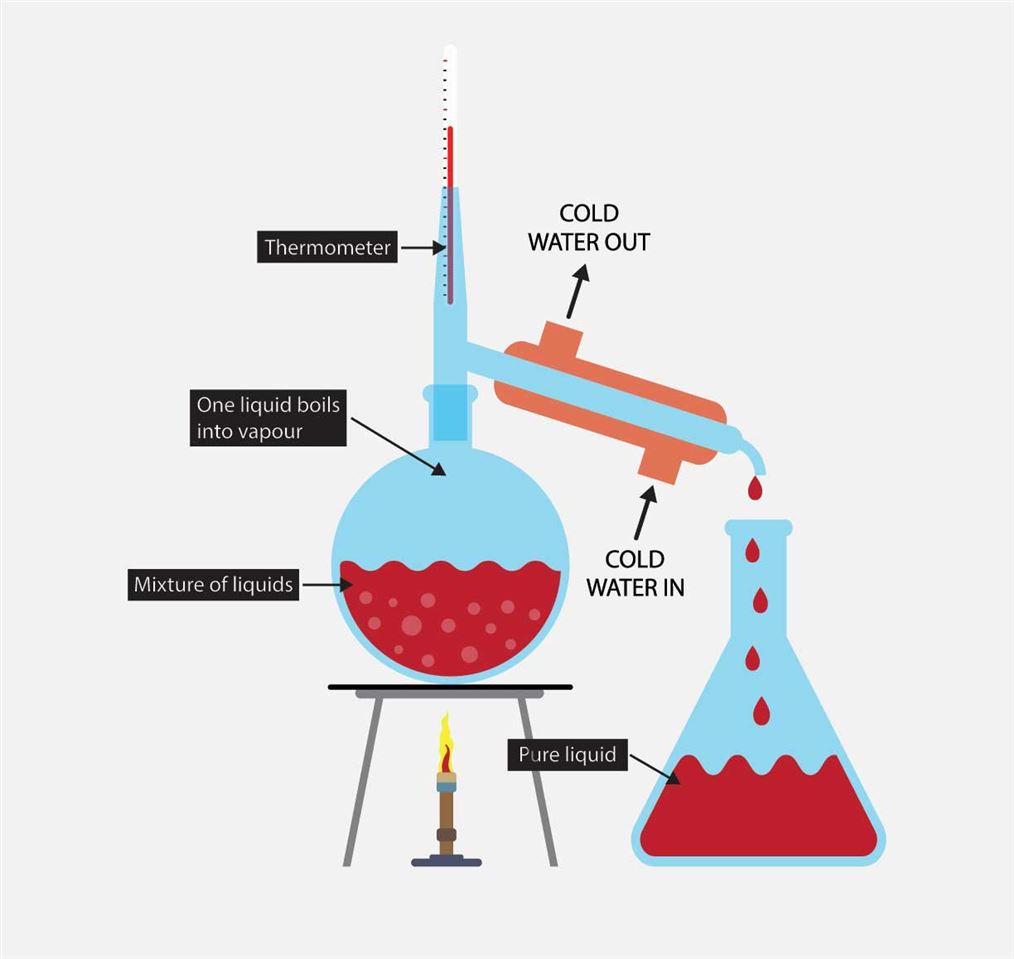
What is Distillation and How is Liquor Made? The People’s Bourbon Review

3 Fractional distillation (Separation of extract) Download Scientific Diagram

EXTRACTION METHODS

Fractional Distillation of Crude Oil RaelynnsrHuff
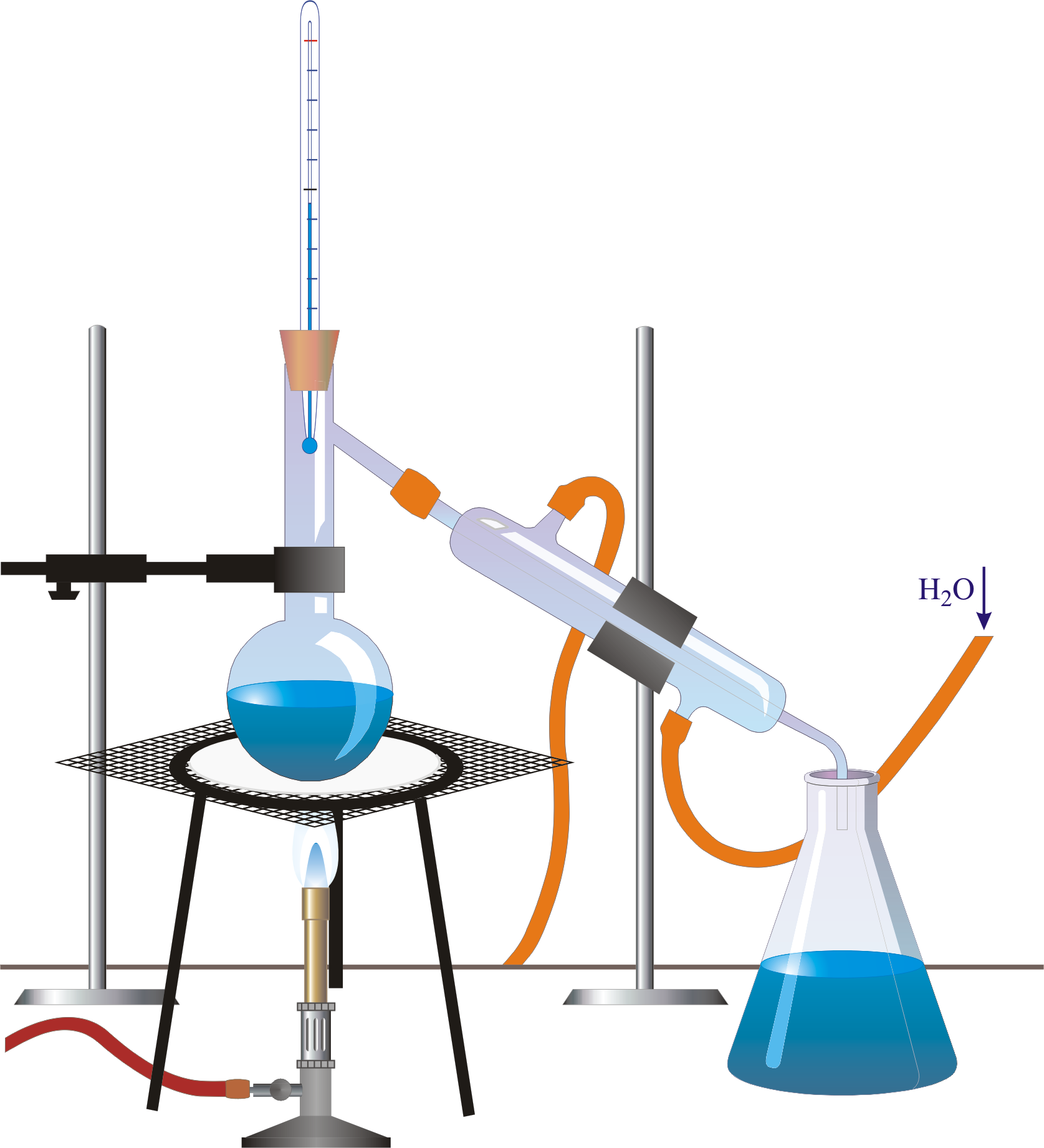
elearningenscr Tous les cours
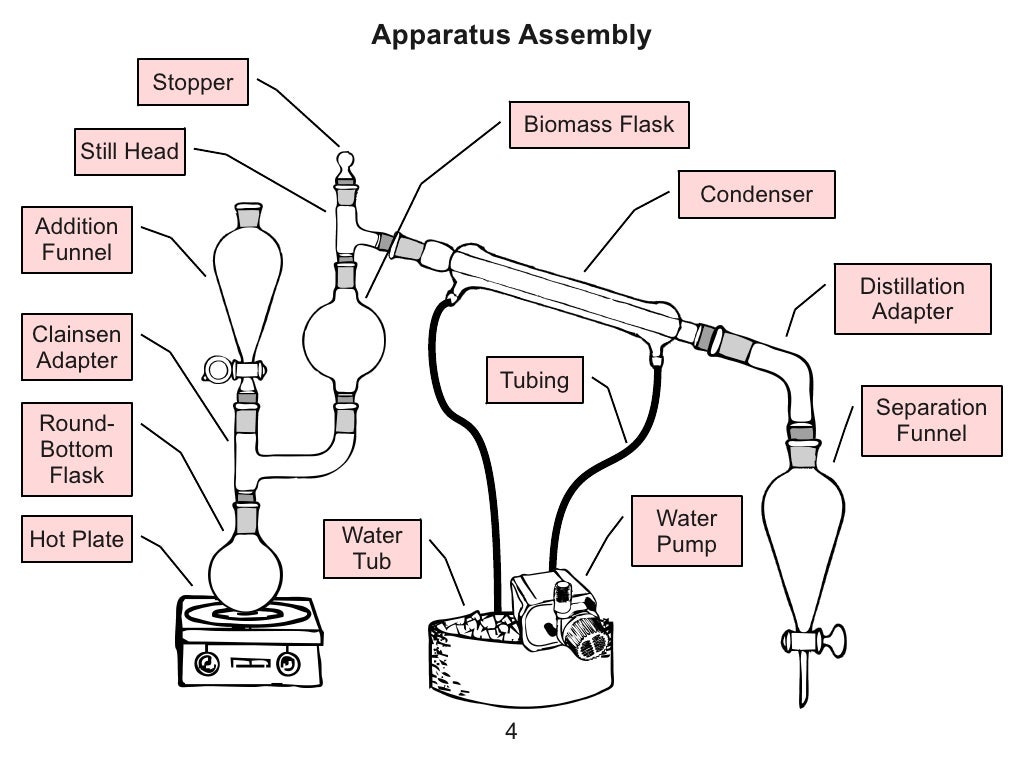
Using Steam Distillation to Produce Essential Oils

Difference Between Distillation and Extraction Process

Green Chemistry Extraction of DLimonene from orange peel

How can we separate a mixture of two miscible liquids A Plus Topper
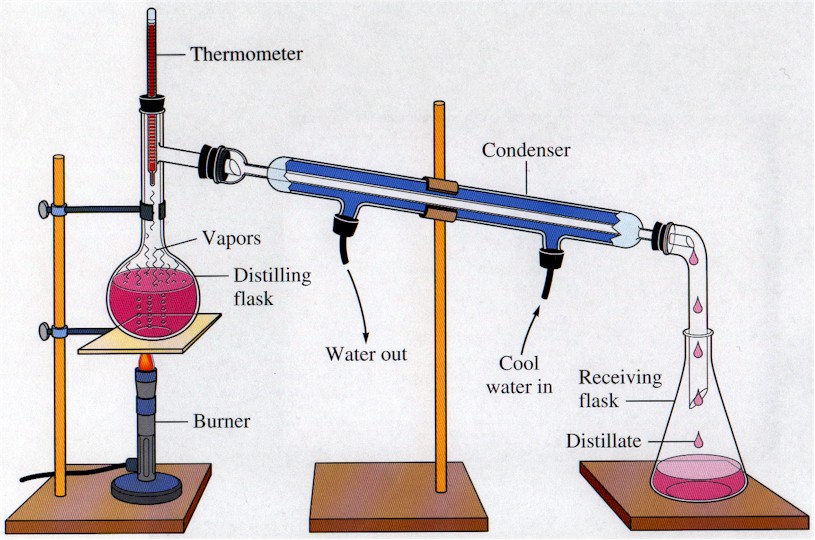
DISTILLATION APPARATUS
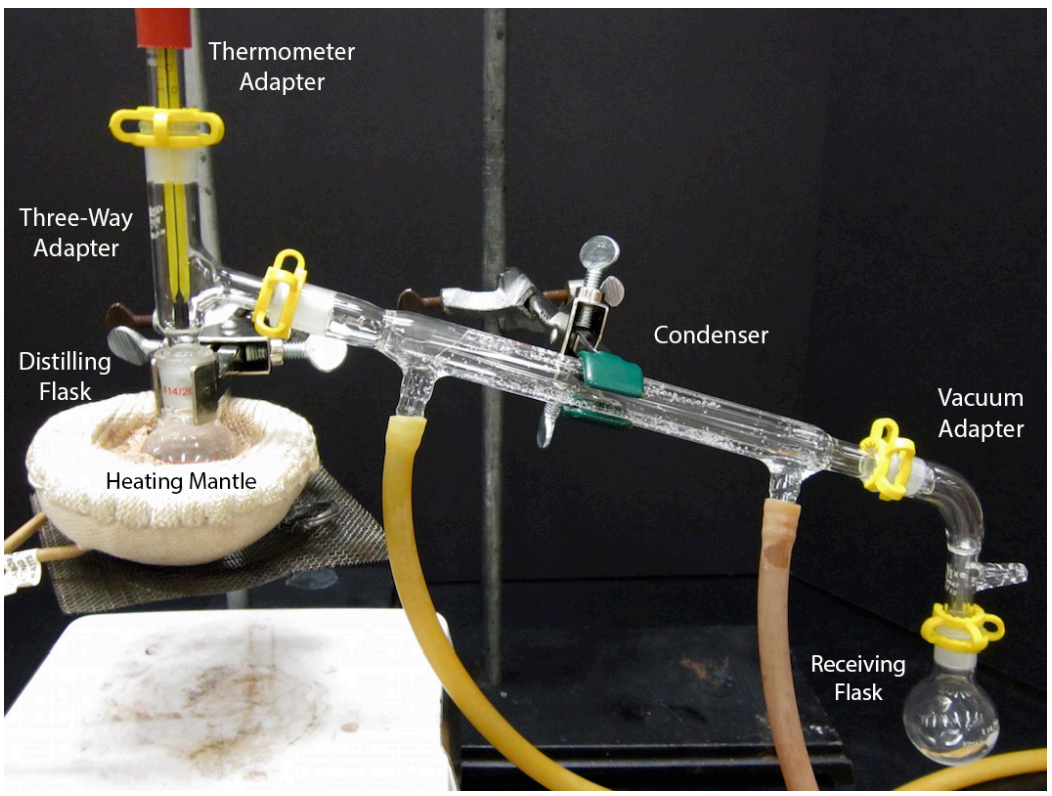
5.2C StepbyStep Procedures Chemistry LibreTexts

Shortpath distillation important distillation equipment for essential oil extraction
Solvent extraction is the most widely used method. The extraction of natural products progresses through the following stages: (1) the solvent penetrates into the solid matrix; (2) the solute dissolves in the solvents; (3) the solute is diffused out of the solid matrix; (4) the extracted solutes are collected.. Azeotropes are also destroyed. The principle arrangement of extractive distillation is very simple (Figure 3). The extractive distillation column shown in Figure 3 consists of three sections: Stripping section, rectifying section and solvent recovery section. The feed material, in this case the benzene fraction, is added between the stripping.


