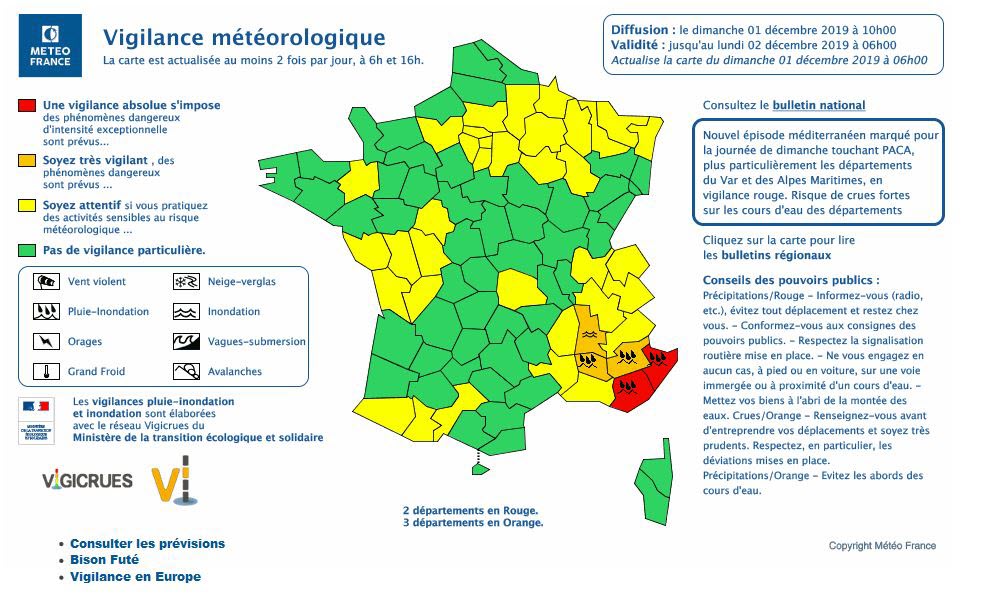
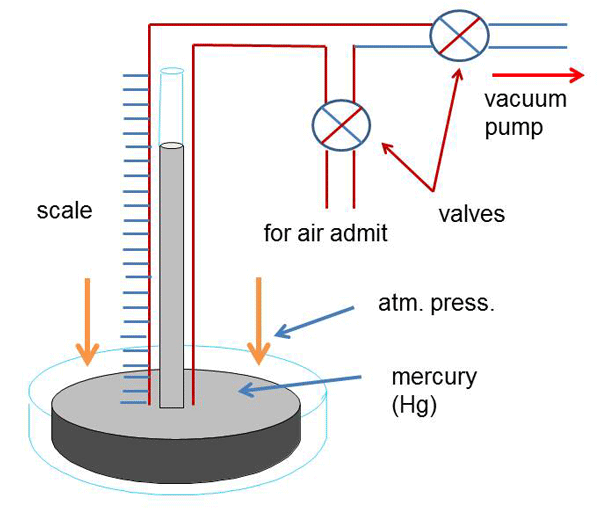
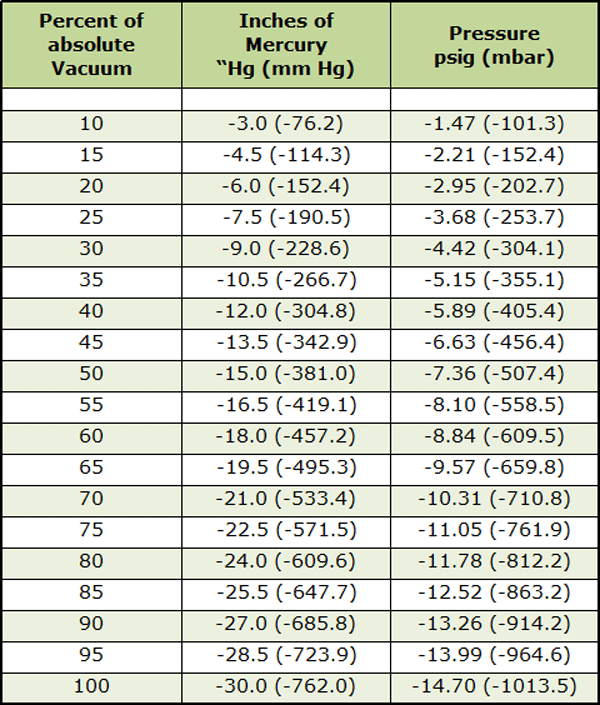
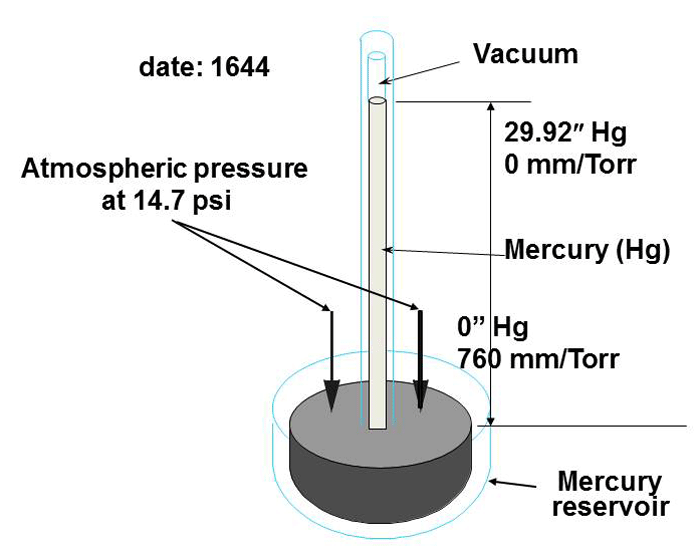
Vacuum levels are commonly measured in terms of in. Hg (inches of mercury), mm Hg (Torr), and microns. These values are derived from an experiment using a bell jar, a clear tube with one end sealed and the other end open, a bowl, and some mercury (see Figure 1 – top). Mercury is poured into the bowl and the tube, filling the tube to the top.. 1 psi (lb/in 2) = 6,894.8 Pa (N/m 2) = 6.895×10-3 N/mm 2 = 6.895×10-2 bar; Download and print Vacuum Units Converter Chart. Convert from % Vacuum to Unit of Pressure. The % of vacuum is a relative value where pressure at normal or standard atmosphere is the base value.. p v% = 100% – (p v / p atm) 100% (1). where
What is a full vacuum in inches of water? Quora
PPT Aerodynamics PowerPoint Presentation, free download ID2321259

Vacuum of up to ten inches of mercury
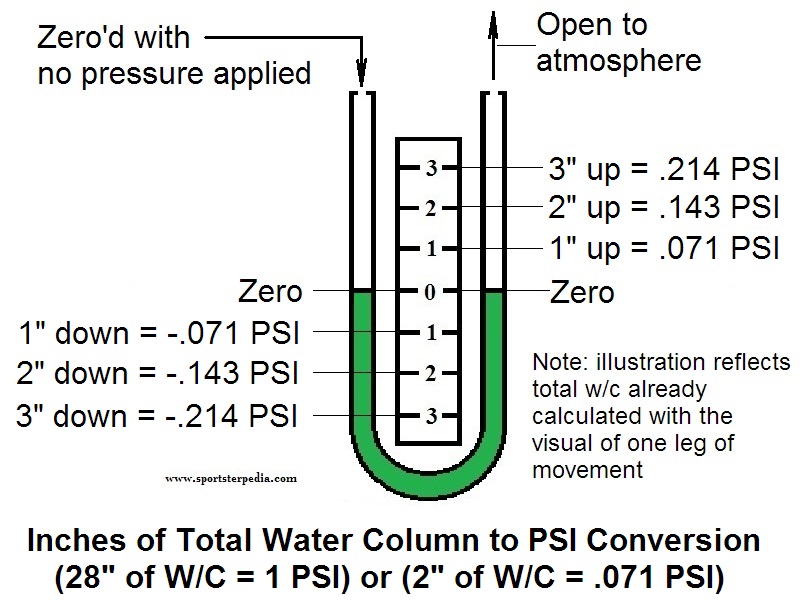
REF GeneralMSR 04 Sportsterpedia
Understanding Vacuum Measurement Units
Mercury Vacuum Gauge Physics Museum The University of Queensland, Australia

Mercury Recovery Vacuum Applied Alloys International

Why is Manifold Pressure Measured in Inches of Mercury (inHG)? Airplane Academy

MV15110PTD 15 Gallon Mercury Recovery Vacuum
Proper Selection and Use of Vacuum Gauges Part One

Nikro Mv00688ss 6 Gallon Mercury Recover Vacuum Mv00688ss Canister Vacuums Vacuum Cleaners
What is a full vacuum in inches of water? Quora

Vacuum Conversion Tables

Inch of mercury Top 6 Facts YouTube
Five Main Reasons for using Vacuum

From Mercury to Digital Various Types of Barometers for Measurement
Industrial Air Controls

Mercury in a vacuum YouTube
Mercury Vacuum Gauge Physics Museum The University of Queensland, Australia
Does a vacuum gauge read in inches of mercury? Quora
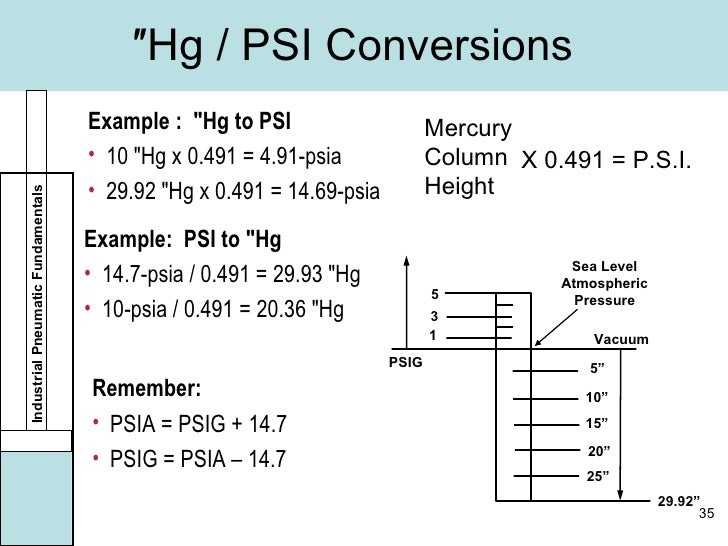
By EngineerExcel. Vacuum pressure refers to the pressure exerted by a vacuum, which is lower than atmospheric pressure. It is commonly measured in units such as torr, pascals, or inches of mercury, and represents the difference between the pressure inside the vacuum and the pressure of the surrounding atmosphere.. Common Vacuum Units and Scales. Millimeters or inches of mercury are still used for measuring pressure in vacuum systems. Millimeters of mercury or mmHg (Hg being mercury in the periodic table of elements) is also a basis for the Torr (after Torricelli) unit of vacuum measurement. 1 Torr equals 1 mmHg and 760 Torr/mmHg equals atmospheric pressure (1 atm).